GX-E4 Long-acting Erythropoesis simulating agent
GX-E4 (Efepoetin alfa)
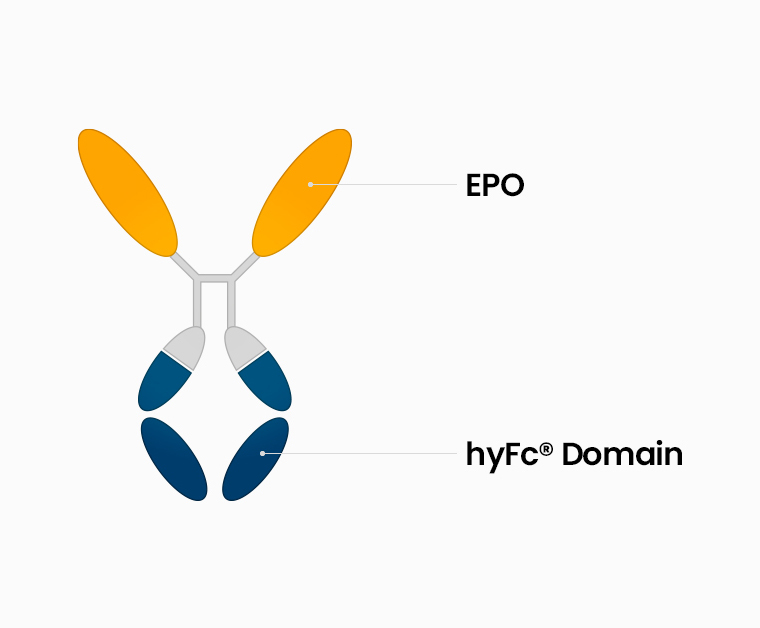
A recombinant human erythropoietin(EPO) fused to hyFc
Disease Indication
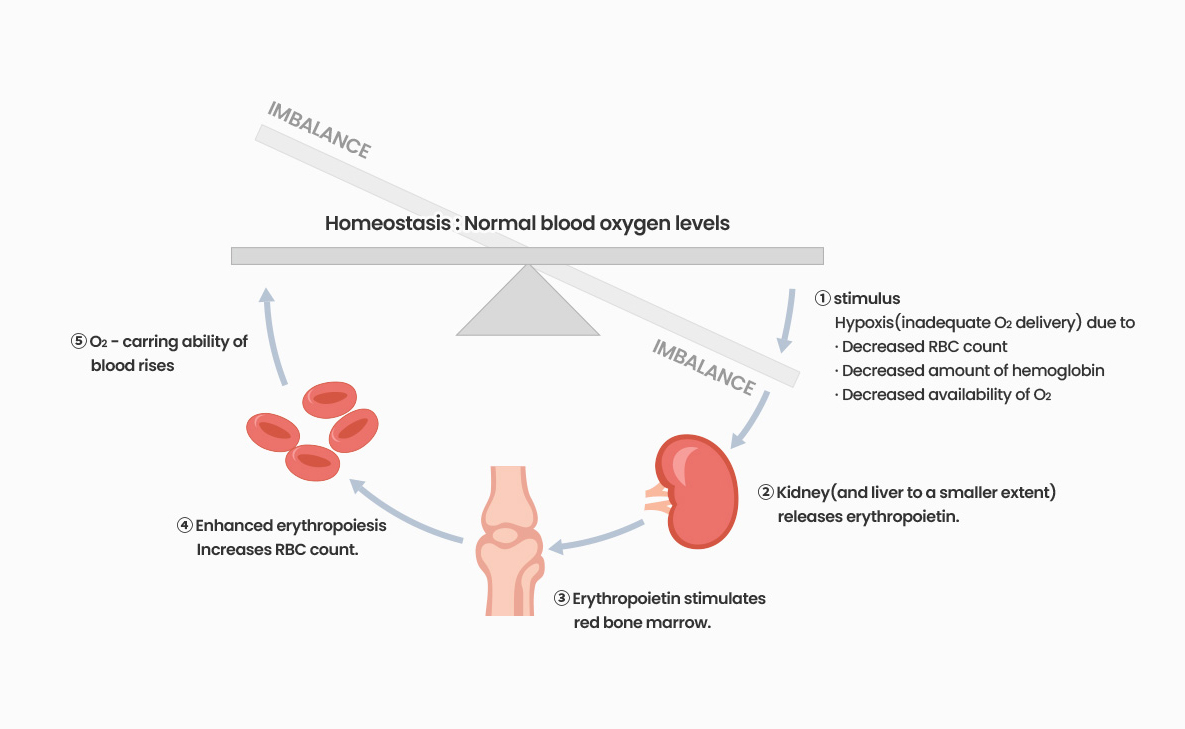
CKD induced Anemia(Dialysis and nondialysis)
Development Stage
Clinical Development (Phase 3)
Safety and tolerability were evaluated from phase 1 clinical trial in healthy volunteers, and phase 2 clinical trial in anemia patients with CKD have been completed and is currently in phase 3 trial with patients in 7 countries in Asia and Oceania.
- 1 Study to evaluate safety, tolerability, and pharmacokinetics/pharmacodynamics of GX-E2 in healthy subjects. NCT02291991 (Phase I, Completed)
- 2 Study to evaluate the efficacy and safety of GX-E2 in anemic patients diagnosed With chronic kidney disease (CKD). NCT02044653 (Phase II, Completed)
- 3 A study of Efepoetin alfa in treating anaemia associated with chronic kidney diseases patient. NCT04155125
Summary
GX-E4 is a Long-acting Erythropoiesis simulating agent. Differing from marketed glycosylation or PEGylation products, it uses the hyFc fusion protein technology to maximize half-life of the efficacy. It is intended for the treatment and for maintenance of anemia induced by chronic kidney disease (CKD) with or without dialysis.
Structure
It has non-cytolytic properties because it uses a sequence that does not have the ability to induce cytotoxicity among the sites of IgG4 and IgD that exist in vivo.
Mechanism
GX-E4 promotes erythropoiesis by interacting with erythropoietin receptors on the red blood cell (RBC) surface, resulting in the proliferation and terminal differentiation of erythroid progenitor cells.
Advantages
- 1Cost effective
- 2Less dosing frequency & longer half-life compared to Epoetin alfa/beta
- 3Non-inferior efficacy & excellent safety profile compared to Aranesp/Mircera
- 4Slow & less fluctuation of Hb levels
Publication
Presentation at 2017 ASN Poster, Presentation at The Korean Society of Nephrology in 2018